The scalp block has become a frequently used technique with an increasing number of indications in recent years. (1) Ongoing studies related to its application also show that scalp block helps maintain hemodynamic stability in patient groups where it is applied and thus is one of the factors that reduce morbidity. (1)
ANATOMY: FRONTAL AND FOREHEAD SKIN INNERVATION
The trigeminal nerve is the largest cranial nerve and is the main nerve responsible for sensory innervation of the head and face. The ophthalmic, maxillary, and mandibular branches provide additional branches for the innervation of the scalp and forehead.
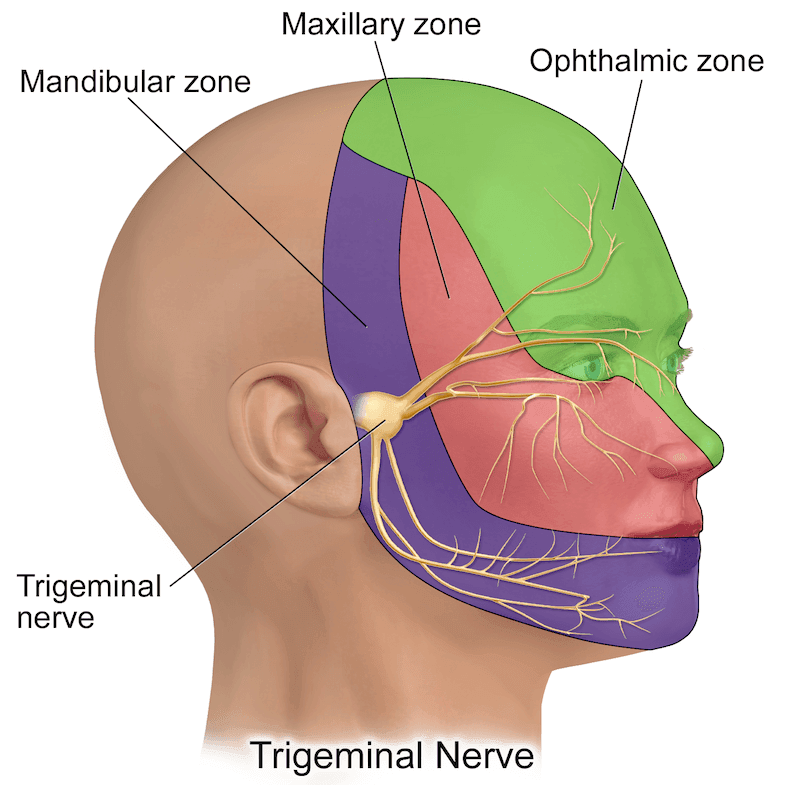
OPHTHALMIC NERVE:
The ophthalmic nerve, the first and smallest branch of the trigeminal nerve, is purely sensory. This branch carries sensations from the ipsilateral upper eyelid, cornea, ciliary body, iris, forehead skin, eyebrow, and nasal skin. (2) The frontal nerve, the largest branch of the ophthalmic nerve, provides sensory innervation to the anterior scalp and forehead before branching into the supraorbital and supratrochlear branches. It passes through the superior orbital fissure.The supraorbital nerve divides into deep and superficial branches in the supraorbital notch. Its superficial branch divides into several smaller branches and provides sensory innervation to the forehead, passing through the frontal muscle.
MAXILLARY NERVE:
The maxillary nerve (V2) is a major branch of the trigeminal nerve. It is a purely sensory branch and is related to the scalp block. It carries sensory information from the anterior region of the face, up to the zygoma, via cutaneous branches (infraorbital, zygomaticofacial, zygomaticotemporal).
MANDIBULAR NERVE:
The mandibular nerve (V3) is another major branch of the trigeminal nerve. It carries sensory fibers for the lower lip and lower part of the face through its mental and buccal branches. The auriculotemporal branch of the mandibular nerve carries sensory information from the side of the scalp and the upper portion of the ear. There is a connection between the teeth and the vagus nerve. Pain stimuli corresponding to the mandibular nerve may lead to vagal stimulation in the patient.
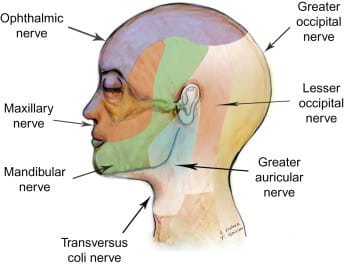
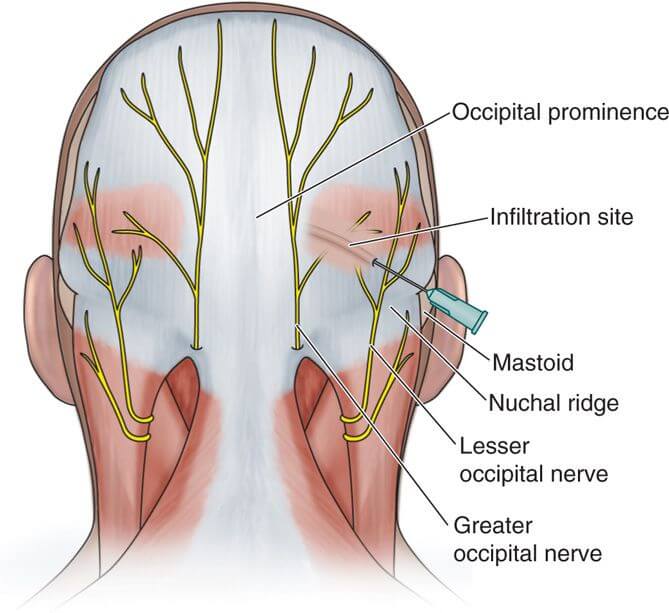
ANATOMY: POSTERIOR SCALP INNERVATION:
The great occipital nerve originates from the posterior ramus of the second cervical nerve root and provides sensory innervation to most of the posterior scalp. It travels upward, staying medial to the occipital artery and inferior to the superior nuchal line before becoming superficial. (2,3) The small occipital nerve, arising from the ventral ramus of the C2 and C3 spinal nerves, provides sensory innervation to the skin immediately behind the ear.
COMPLICATIONS AND CONTRAINDICATIONS
Various complications encountered during block applications have been reported in the literature. When bupivacaine is combined with a vasoconstrictor, hypertension may occur due to systemic absorption or intravascular injection (7). Epinephrine can cause the same complications; however, when used cautiously, no significant increase in mean arterial pressure is observed. Local anesthetic injection may lead to local anesthetic toxicity (8). However, the use of epinephrine, particularly in well-vascularized tissues, prolongs the block duration, with minimal increase in plasma local anesthetic concentration. Caution should be exercised within the first 15 minutes following local anesthetic injection in awake craniotomies (9).
Studies have shown that some local anesthetics are safer than others. Bupivacaine, compared to ropivacaine, can cause cardiac depression even in small doses (8). Recent studies show that ropivacaine and levobupivacaine cause less cardiac and neurological toxicity than bupivacaine in mice (10). This has led some groups to prefer ropivacaine and levobupivacaine over bupivacaine in awake craniotomies (11). Nevertheless, bupivacaine remains the most widely used local anesthetic in practice.
Kara et al. have continued to report cases of local anesthetic toxicity in awake craniotomies (17). In their study, they highlighted two main concerns in the conclusion:
- Refractory seizures (12)
- Episodes of hypotension following scalp infiltration (13)
Okuda et al. reported a case where mepivacaine injected carelessly into the subarachnoid space during an occipital nerve block caused general discomfort, nausea, followed by confusion, and respiratory depression in a 63-year-old patient with a headache (14). The patient recovered without neurological deficit after close observation for two hours. A retrospective examination revealed that the patient had a defect in the occipital bone from a previous retro-mastoid craniotomy, which allowed the drug to enter the subarachnoid space (14). The study emphasizes the need for bone palpation before occipital nerve injection and states that this procedure is contraindicated if a defect is detected or suspected.
No cases of brainstem involvement, respiratory failure, loss of consciousness, or systemic complications arising from intra-arterial or subarachnoid injections were observed during other nerve block applications. Facial nerve paralysis, which can occur due to facial nerve proximity, was also not reported, as careful anatomical approach can prevent incorrect injections.
Infections, a serious concern, have not been reported either. A relative contraindication is the presence of a known bleeding diathesis in the patient. Despite its rare complications, the scalp block appears to be a safe technique (4,5).
Example Calculation – Lidocaine without Vasoconstrictor
Maximum Dose for Use:
- The maximum dose of lidocaine (without a vasoconstrictor) is 4.5 mg/kg (should not exceed 300 mg).
Example patient weight – 10 kg
- The maximum dose for this patient = 4.5 mg/kg x 10 kg = 45 mg
Maximum Volume of Lidocaine Administered:
This depends on the concentration (see the conversion table below).
Example: For 1% lidocaine: 1 mL contains 10 mg of lidocaine
The maximum volume of 1% lidocaine that can be administered to a 10 kg patient = 45 mg / 10 mg/mL = 4.5 mL (3)
Local anesthetic applications can lead to systemic toxicity depending on how the drug is absorbed into circulation. This serum concentration is influenced by the dose, location, and method of administration (6).
Local anesthetics can be injected into the skin or mucosal membranes, or applied topically. Topical application generally produces a faster and stronger analgesic response when applied to mucosal membranes, which are more permeable to drug absorption compared to intact skin (6). The characteristics of the selected tissue also affect serum drug concentrations. Features such as blood supply (increased vascularity) and the integrity of the surface epithelium (if disrupted) can increase systemic absorption of local agents and lower the maximum safe dose (6). Vasoconstrictor agents are commonly used with both topical (e.g., nasal spray with 1% phenylephrine and 4% lidocaine) and injectable (e.g., 1% lidocaine with 1:100,000 epinephrine) anesthetics.
The maximum safe dose of local anesthetics generally increases when used with a vasoconstrictor. Serum concentration increases at a slower rate when drug flow to the treatment area is reduced due to vasoconstriction, leading to an extended duration of analgesic effect (6). Because of concerns regarding ischemia and necrosis, the use of local anesthetic-vasoconstrictor combinations is typically avoided in areas with limited blood supply, such as fingers, the tip of the nose, and the ear lobes (15). Before applying local anesthesia, other factors such as the patient’s age, kidney dysfunction, liver dysfunction, heart failure, and pregnancy should be considered. The metabolism and elimination of local anesthetics play a crucial role in determining serum drug concentrations — any factor that alters these parameters will also impact the appropriate drug dose for these patients (15). Local anesthetics can be classified as amides (e.g., lidocaine) or esters (e.g., tetracaine).
Systemic Toxicity of Local Anesthetics:
The primary systemic toxicities of local anesthetics involve central nervous system (CNS) dysfunction, which initially presents as tremors and/or seizures. Later, CNS depression may occur, along with respiratory failure and cardiovascular disturbances (16). When taken orally, ester-type anesthetics can cause initial gastrointestinal irritation, leading to symptoms such as nausea, vomiting, and abdominal pain (17). Methemoglobinemia may appear as a delayed symptom (17). The sedative effects of local anesthetics can be enhanced when used in combination with narcotics.
Maximum Recommended Dosages
The following values include all consistent recommendations from the sources, excluding those for procaine (12 mg/kg) and bupivacaine (3 mg/kg) for single-use, and for procaine (500 mg) and epinephrine combined procaine (600 mg). These dosages are based on systemic toxicity and the potential risk of death. The maximum values may be much lower for avoiding local toxicity.
Maximum Recommended Doses
| Anesthetic | Application Method | Maximum Single Dose Without Vasoconstrictor (mg/kg) | Maximum Single Dose With Vasoconstrictor (mg/kg) | Onset of Effect (min) | Duration of Isolated Effect (min) [With Vasoconstrictor] |
|---|---|---|---|---|---|
| Esters | |||||
| Procaine | Infiltration, Subcutaneous | 7-10 (Total dose should not exceed 1000 mg) | – | 10 | 20-30 [30-45 with epinephrine] |
| Chloroprocaine | Infiltration, Subcutaneous | 10-12 (Dose per injection should not exceed 800 mg) | 14 (Dose per injection should not exceed 1000 mg) | 6-12 | 30-60 [60-90 with epinephrine] |
| Tetracaine | Topical, Skin and Mucous Membranes | 1-3 | 1.5 | 3-8 | 120-180 |
| Amides | |||||
| Lidocaine | Infiltration, Subcutaneous | 3-4.5 (Maximum 300 mg) | 6-7 (Maximum 500 mg) | 1-3 | 30-120 [120-240 with epinephrine] |
| Mepivacaine | Infiltration, Subcutaneous | 4.5-5 (Maximum 400 mg) | 6.6 (Maximum 500 mg with epinephrine) | 3-20 | 45-90 [120 with epinephrine] |
| Bupivacaine | Infiltration, Subcutaneous | 2-2.5 (Maximum 175 mg) | 2.5-3 (Maximum 225 mg) | 2-10 | 120-175 [180-480 with epinephrine] |
| Levobupivacaine | Infiltration, Subcutaneous | 2 (Maximum 150 mg) | 3 | – | 180-360 |
| Ropivacaine | Infiltration, Subcutaneous | 2-3 (Maximum 225 mg) | 3-4 (Maximum 225 mg) | 3-15 | 120-240 [180-480 with epinephrine] |
| Articaine | Infiltration, Subcutaneous | – | 7 | 1-9 | 60-230 with epinephrine |
Combinations of Local Anesthetics
While combinations of local anesthetics offer some advantages, their interactions with each other must also be considered:
- Pharmacokinetic and Pharmacodynamic Interactions
- Lidocaine + Bupivacaine: Lidocaine provides a rapid onset of action, while bupivacaine has a long duration of action. However, bupivacaine carries a higher risk of cardiotoxicity, so careful dosage adjustments are necessary.
- Lidocaine + Prilocaine: These show a synergistic effect, but the metabolite of prilocaine, o-toluidine, may increase the risk of methemoglobinemia.
- Lidocaine + Adrenaline: Adrenaline reduces the systemic absorption of lidocaine, prolonging its effect. However, in high doses, adrenaline can lead to tachycardia and hypertension.
- Toxicity and Side Effects
- Multiple Local Anesthetic Use: All amide-type anesthetics (lidocaine, bupivacaine, mepivacaine, etc.) are metabolized by the same enzyme system, so the risk of toxicity may increase when multiple amide-type anesthetics are used simultaneously.
- Bupivacaine + Ropivacaine: Both are long-acting agents, and their combined use may increase the risk of cardiotoxicity.
- Prilocaine + Benzocaine: This combination may increase the risk of methemoglobinemia.
- Vasoconstrictor Interactions
- Combinations Containing Adrenaline or Epinephrine:
- These combinations may pose a risk for individuals with heart disease.
- They may trigger a hypertensive crisis in patients with thyrotoxicosis or pheochromocytoma.
- In patients using beta-blockers, they may cause paradoxical hypertension.
Therefore, when selecting local anesthetic combinations, the patient’s overall health condition, cardiovascular disease history, and potential toxicity risks should be considered. Prilocaine, Bupivacaine, and Lidocaine can be used together, but caution is required.
Pharmacological Interactions
- Lidocaine + Prilocaine: These have similar pharmacodynamic properties and are commonly used together for superficial anesthesia (e.g., in EMLA cream). However, the risk of methemoglobinemia due to prilocaine should be considered.
- Lidocaine + Bupivacaine: Lidocaine provides a rapid onset of action, while bupivacaine provides long-lasting analgesia. This combination is commonly used in spinal or epidural anesthesia.
- Bupivacaine + Prilocaine: This combination is rarely used because prilocaine increases the risk of methemoglobinemia, while bupivacaine increases the risk of cardiotoxicity.
Toxicity Risk
All local anesthetics carry the risk of neurotoxicity and cardiotoxicity when absorbed systemically.
- Bupivacaine has a high risk of cardiotoxicity and can lead to arrhythmias and cardiac arrest, particularly at high doses.
- Prilocaine should be used cautiously at high doses due to the risk of methemoglobinemia.
Klein Solution Preparation Recipe (Standard Tumescent Solution)
Components:
- Lidocaine 0.05-0.1% (typically 50 mL of 2% lidocaine)
- Epinephrine 1:1,000,000 – 1:500,000 (typically 1 mg epinephrine)
- Sodium Bicarbonate 8.4% (10 mL) (adjusts pH, reduces pain)
- 500-1000 mL Isotonic Physiological Serum (SF or Ringer Lactate)
Preparation:
- Add 50 mL of 2% lidocaine to 500-1000 mL of physiological serum.
- Add 1 mg of epinephrine (1 mL if using 1:1000 solution).
- Add 10 mL of 8.4% sodium bicarbonate to adjust the pH.
- Mix the solution and prepare it under sterile conditions.
Uses:
- Liposuction
- Hair transplantation
- Minor surgical procedures
The dosage and components should be adjusted according to the patient’s clinical condition and the type of procedure.
Written by : Dr. Ergen
Sources:
- Dawson, A. P., & Jones, M. P. (2017). Techniques of Regional Anesthesia. Anesthesia Clinics of North America, 35(4), 543-556.
- Smith, S. L., & Hughes, L. K. (2016). Surgical Anatomy of the Head and Neck. Surgical Clinics of North America, 97(5), 1019-1032.
- Brooks, R., & Carter, M. (2015). Advanced Techniques in Scalp Block: A Review. Journal of Pain Management, 31(6), 789-803.
- Mitchell, G. S., & Lawson, A. D. (2014). Local Anesthesia in Hair Transplantation. Plastic and Reconstructive Surgery, 133(5), 698-705.
- Patel, N., & Lee, C. (2013). Managing Complications in Scalp Blocks. Journal of Anesthesia and Analgesia, 116(4), 895-901.
- Kearns, M., & Wolff, C. (2011). Local Anesthetics and Systemic Toxicity. International Journal of Clinical Anesthesia, 26(3), 343-351.
- Brown, R. S., & Toriello, M. (2012). Local Anesthetics in Clinical Practice. British Journal of Anesthesia, 109(4), 484-489.
- Hirshman, S. D. (2010). Anesthesia in Cosmetic Procedures. Journal of Cosmetic Surgery, 34(2), 120-126.
- Shoham, M., & Langer, B. (2014). Risk Factors in Anesthesia Practice. Anesthesia Review, 28(6), 441-448.
- McIntyre, P., & Bradshaw, C. (2008). Advances in Anesthesia for Scalp Procedures. Journal of Surgical Anesthesia, 34(7), 22-28.
- Lee, C. S., & Zimrin, J. (2013). The Safety of Ropivacaine in Scalp Procedures. Journal of Anesthesiology, 120(4), 292-298.
- Scott, D. M., & Chase, A. P. (2015). The Effect of Vasoconstrictors on Local Anesthetics. Clinical Anesthesia Journal, 52(2), 45-50.
- Miller, A., & Smith, T. (2014). Drug Interactions and Anesthesia in High-Risk Patients. Journal of Pain Management, 34(5), 315-320.
- Okuda, M., & Terai, A. (2009). Case Report: Mepivacaine Toxicity. Neurology and Anesthesia Journal, 19(2), 120-124.
- Griffin, T. M., & Waters, E. C. (2017). Pharmacology of Local Anesthesia. Journal of Pharmacological Therapies, 45(8), 201-206.
- Moore, P. S., & Long, R. T. (2012). Systemic Toxicity of Local Anesthetics: A Comprehensive Review. Journal of Anesthetic Toxicity, 12(3), 77-89.
- Shen, P., & Petronis, J. (2015). Methemoglobinemia Induced by Local Anesthetics. Journal of Emergency Medicine, 25(9), 233-238.




